Drug Development Overview
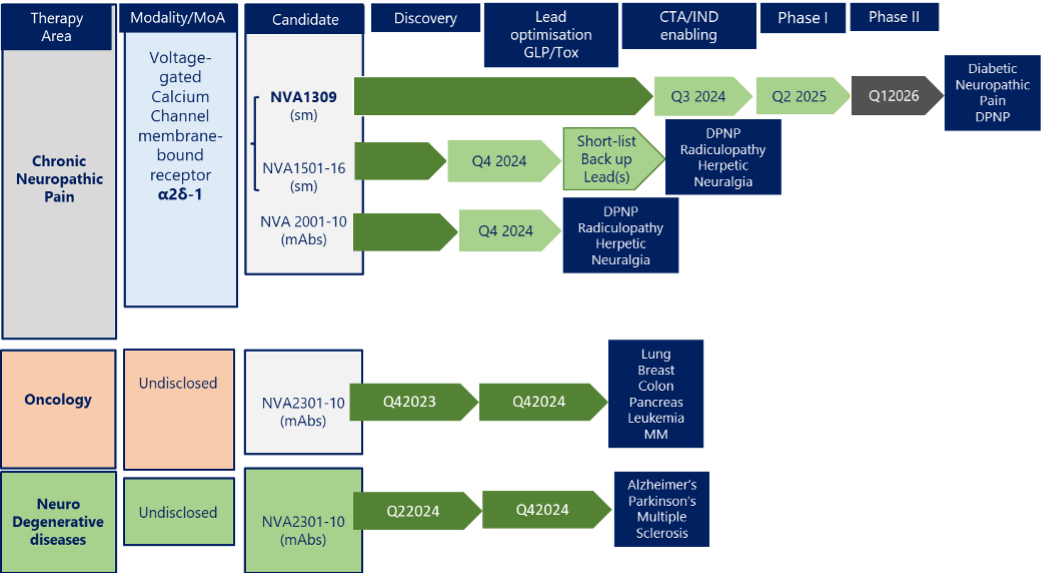
NVA1309 is being developed for Chronic Neuropathic Pain, currently at the GLP/Tox stage. NVA1309 has unique properties to include, non-brain penetration (but spinal cord penetration) peripheral nerve localization and α2δ-1 versus α2δ-2 specificity. These features together with its 10x higher potency over pregabalin make it a promising drug candidate for a broad range set of debilitating pain conditions such as diabetic painful neuropathic pain (DPNP), post-surgical pain (PSP), post-herpetic neuralgia (PHN), lumbar radiculopathy (sciatica, LR) and fibromyalgia.
Our pipeline also includes 16 (NVA1501-16) additional small-molecule compounds to be explored for activity in neuropathic pain as well as monoclonal antibodies against the α2δ-1 to be developed as potential therapeutics in neuropathic pain and solid tumors.
NVA 2301-10 are a series of humanized monoclonal antibodies currently in development for Oncology indications as well as for Alzheimer’s and Parkison’s diseases and are in early discovery MoA/lead optimization stage.
Drug Development Pipeline